Experimental and theoretical studies on thymine photodimerization mediated by oxidatively generated DNA lesions and epigenetic intermediates†
Abstract
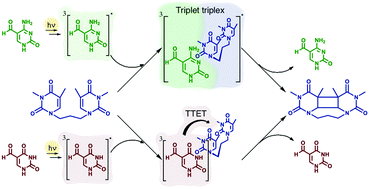
Interaction of nucleic acids with light is a scientific question of paramount relevance not only in the understanding of life functioning and evolution, but also in the insurgence of diseases such as malignant skin cancer and in the development of biomarkers and novel light-assisted therapeutic tools. This work shows that the UVA portion of sunlight, not absorbed by canonical DNA nucleobases, can be absorbed by 5-formyluracil (ForU) and 5-formylcytosine (ForC), two ubiquitous oxidatively generated lesions and epigenetic intermediates present in living beings in natural conditions. We measure the strong propensity of these molecules to populate triplet excited states able to transfer the excitation energy to thymine–thymine dyads, inducing the formation of cyclobutane pyrimidine dimers (CPDs). By using steady-state and transient absorption spectroscopy, NMR, HPLC, and theoretical calculations, we quantify the differences in the triplet–triplet energy transfer mediated by ForU and ForC, revealing that the former is much more efficient in delivering the excitation energy and producing the CPD photoproduct. Although significantly slower than ForU, ForC is also able to harm DNA nucleobases and therefore this process has to be taken into account as a viable photosensitization mechanism. The present findings evidence a rich photochemistry crucial to understand DNA damage photobehavior.


 Please wait while we load your content...
Please wait while we load your content...