New insight into electrosynthesis of ordered TiO2 nanotubes in EG-based electrolyte solutions: combined experimental and computational assessment†
Abstract
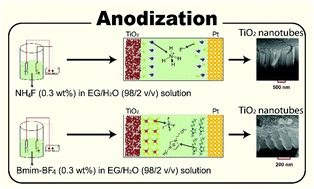
To obtain a better understanding of TiO2 nanotube (TiO2-NT) synthesis in different ethylene glycol (EG)-based electrolyte solutions by electrochemical anodization, the primary steps of TiO2-NT formation were studied by experimental techniques. In this regard, three different EG-based electrolyte solutions were used for anodic oxidation of titanium foil. The first electrolyte solution contains conventional ammonium fluoride (NH4F) dissolved in EG/water (98 : 2 v/v). In the second one, Ti foil anodization is performed in an electrolyte solution containing the 1-butyl-3-methyl-imidazolium tetrafluoroborate (Bmim-BF4) ionic liquid. Finally, the fluorine-containing species was replaced by the 1-butyl-3-methyl-imidazolium chloride (Bmim-Cl) ionic liquid. The results indicate that the TiO2-NTs did not form by anodization in the EG/H2O/Bmim-Cl electrolyte solution at 60 V. Interestingly, this electrolyte solution is less viscous than the fluorine-containing electrolyte solutions. In addition, we report a detailed study on the structural arrangement of electrolyte solution components near the solid surfaces using molecular dynamics (MD) simulation methods to reveal the factors governing the difference of the ionic species distribution. The MD results elucidate the role of the ionic constituents in the length of the nanotube arrays at a certain anodization condition. Furthermore, as reported herein for the first time, the lifetimes of ion–ion contacts and the interactions of ionic species with TiO2 walls have a substantial effect on the resulting nanotubes. These characteristics are analyzed by using radial distribution functions, density profiles, distance analysis, time correlation functions, and mean-square-displacements, complemented by DFT calculations.


 Please wait while we load your content...
Please wait while we load your content...