Rational design of photo-cleavable alkoxyamines for polymerization and synthesis†
Abstract
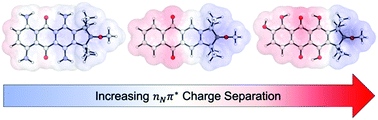
Theoretical calculations have been performed in order to investigate the impact of different substitution patterns on predicted photoreactivity of alkoxyamines fused to an anthraquinone chromophore. Amino and hydroxy groups (similar to those which have been previously synthesized) are introduced and their effect on excited state energies and charge transfer is assessed. Analogous to formally oxidized alkoxyamines, the charge-separated nNπ* state can undergo mesolytic cleavage or bimolecular or SN2 reactions with nucleophiles, according to the substitution patterns and other reagents present. While homolytic cleavage is in principle promoted by triplet ππ* states, the accessible ππ* triplet states in this system are centered on the chromophore and unreactive. We show that the reactive nNπ* state, which bears a negative charge, is stabilized by hydroxy substitution while amino substitution will destabilize it. After mesolysis to a carbon centred radical, the nitroxide radical re-forms; however, when carbocations are produced the remaining open-shell singlet is stable and unable to undergo coupling with the carbocation.


 Please wait while we load your content...
Please wait while we load your content...