Understanding the electrochemical properties of bulk phase and surface structures of Na3TMPO4CO3 (TM = Fe, Mn, Co, Ni) from first principles calculations†
Abstract
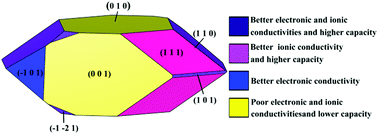
First principles calculations were performed to investigate the electrochemical performance (voltage, cycling stability, electrical conductivity, mechanical properties and safety) of the bulk phase and surface structures of Na3TMPO4CO3 (TM = Fe, Mn, Co, Ni). Na3FePO4CO3 and Na3MnPO4CO3 are estimated to be promising candidates for the cathode materials of sodium ion batteries because of the moderate voltages, good stability and high safety during the cycling process of two sodium ions per formula unit. For the purpose of improving the rate performances, Na3MnPO4CO3 was chosen as an example to explore its surface performance. The surface energies, equilibrium morphology, redox potentials and electronic conductivities of surfaces are explored in detail. The results suggest that (010), (001), (111) and (110) orientations are the dominating surfaces in the Wulff shape, while the surfaces (010) and (001) possess high second surface redox potentials, corresponding to the unsatisfactory specific capacity and ionic conductivity. Moreover, low surface band gaps are discovered in all orientations, which gives a good explanation for the enhanced electronic conductivity as a consequence of decreasing particle size. In addition, the (110), (101) and (12−1) surfaces display significantly lower surface band gaps and comparatively lower second redox potentials, thus enlarging the relative surface areas of surfaces (110), (101) and (12−1) could be an efficient methodology to further improve the specific capacity and electronic conductivity of the Na3MnPO4CO3 material.


 Please wait while we load your content...
Please wait while we load your content...