Oxygen K-shell spectroscopy of isolated progressively solvated peptide†
Abstract
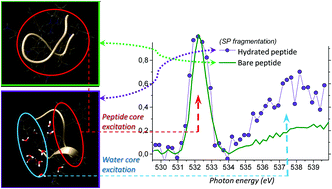
Gas-phase near-edge X-ray-absorption fine structure (NEXAFS) action spectroscopy around the oxygen K-edge and mass spectrometry were employed to probe isolated substance P (SP) molecular ions, both bare and progressively solvated with 4 and 11 water molecules. Detailed mass spectra of bare and hydrated precursors are presented for the resonant photon energy of 532 eV that corresponds to O1s → π(amide)* core excitation, triggering resonant Auger decay and fragmentation from the ionized radical molecular system. The fragmentation pattern of doubly protonated SP hydrated with 4 water molecules clearly shows a series of abundant doubly charged backbone fragments, as well as triply charged precursor with small neutral losses, all preserving full water cluster. This is drastically different from the collisional induced dissociation of the hydrated peptide where the water loss is a dominant relaxation process. Moreover, the action NEXAFS obtained from several resolved small backbone fragments revealed increased fragmentation of hydrated SP relative to the bare one, due to a resonant O1s excitation of the attached water molecules. Such unexpected result inspires further experimental developments to investigate possible nonlocal energy transfer from the solvent to the biomolecules within the first solvation shell. The experiment is supported by molecular dynamics and DFT calculations to estimate the intensity of the resonant X-ray absorption of bare and hydrated SP around peptide and water O1s excitation region.


 Please wait while we load your content...
Please wait while we load your content...