Theoretical formulation of Li3a+bNaXb (X = halogen) as a potential artificial solid electrolyte interphase (ASEI) to protect the Li anode†
Abstract
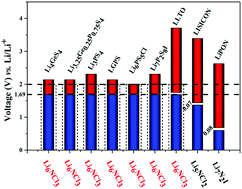
A major problem against the realization of high energy density and safe solid Li-ion batteries lies in detrimental reactions at the interface between the lithium anode and the solid electrolytes. This makes it necessary to develop an artificial solid electrolyte interphase (ASEI) as an effective protective coating to the lithium anode, which is the “Holy Grail” to enable high energy density batteries owing to its extremely high capacity. Here in this work, we carried out high-throughput first-principles modelling in the framework of materials genome engineering to identify potential ASEIs based on lithium nitric halides Li3a+bNaXb (X: halogen F, Cl, Br, I). On the basis of comprehensive assessments covering material stability, ionic conductivity, elastic modulus, and electrochemical compatibility with Li and potential SSEs, we have identified lithium nitric halides such as Li6NCl3 as superb ASEI candidates in line with their adequate ionic conductivity and fairly wide electrochemical window from 0 to 2 V (vs. Li/Li+). This makes them compatible with the lithium anode and various well-recognized thiophosphate SSEs such as LGPS, Li6PS5Cl, and Li3PS4, which have typical reduction potentials around 1.71 V. Besides, the large elastic modulus (e.g. 61.12 GPa for Li6NCl3) could be highly effective against the formation of lithium dendrites.


 Please wait while we load your content...
Please wait while we load your content...