Asymmetric synthesis of benzothiazolopyrimidines with high catalytic efficiency and stereoselectivity under bifunctional phosphonium salt systems†
Abstract
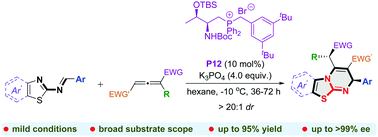
An efficient formal [4+2] annulation between 2-benzothiazolimines and allenoates mediated by an amino acid-derived bifunctional phosphonium salt catalyst is developed. This protocol provides a new and facile synthetic approach to create a broad range of isothiourea-based benzothiazolopyrimidine derivatives under mild reaction conditions with high isolated yields and excellent diastereo- and enantioselectivities.


 Please wait while we load your content...
Please wait while we load your content...