Asymmetric acyl-Mannich reaction of isoquinolines with α-(diazomethyl)phosphonate and diazoacetate catalyzed by chiral Brønsted acids†
Abstract
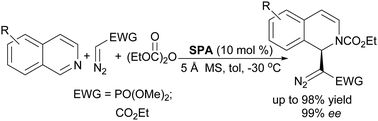
An efficient asymmetric acyl-Mannich reaction of isoquinolines with α-(diazomethyl)phosphonate and diazoacetate has been developed using chiral spiro phosphoric acids as catalysts. This reaction allowed the construction of a series of chiral 1,2-dihydroisoquinolines bearing a tertiary stereocenter at the C1 position with up to 98% yield and 99% ee.


 Please wait while we load your content...
Please wait while we load your content...