Metal-free spirocyclization of N-arylpropiolamides with glyoxylic acids: access to complex azaspiro-fused tricycles†
Abstract
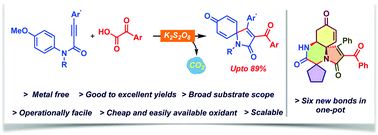
A metal-free oxidative cascade acylation and dearomatization of N-(p-methoxyaryl)propiolamides was achieved via K2S2O8 mediated decarboxylation of α-oxocarboxylic acids under operationally simple conditions to access azaspiro[4,5]-trienones in good to excellent yields. Furthermore, the utility of the protocol was illustrated in a one-pot reaction sequence consisting of Ugi-reaction/spirocyclization/aza-Michael transformation for the construction of complex tricyclic cores having quaternary spirocenters.


 Please wait while we load your content...
Please wait while we load your content...