Visible light-mediated Smiles rearrangements and annulations of non-activated aromatics†
Abstract
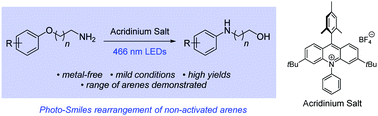
We report the first examples of radical cation Smiles rearrangements. A series of aryloxy alkylamines underwent spontaneous reaction, with the amino group displacing the ipso-alkoxy group through substitution, at ambient temperature and under photoactivation by visible light in the presence of an acridinium catalyst (5 mol%). The study was extended to 3-(2-methoxyphenyl)propan-1-amine derivatives, which lack an appropriate ipso leaving group. Here, efficient cyclisations resulted in displacement of the methoxy group and formation of tetrahydroquinolines.


 Please wait while we load your content...
Please wait while we load your content...