Adaptation of thermophilic acetyltransferase to a water-mediated catalytic mechanism†
Abstract
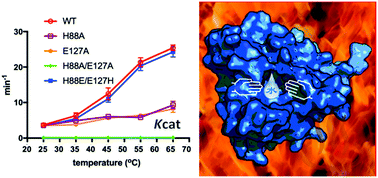
The common mechanism of N-acetyltransferases (NATs) is a water-mediated catalysis, which is not conducive to thermophilic acetyltransferases. The crystal structure of SsArd1 shows an ordered catalytic water molecule in a trap formed by the residues H88 and E127. Structure-guided mutagenesis, kinetic studies and MD simulation indicated that the turnover rates of H88A, E127A and H88A/E127A mutants were low, but that of the H88E/E127H mutant could be restored to the level of the wild type.


 Please wait while we load your content...
Please wait while we load your content...