On-demand acid-gated fluorescence switch-on in photo-generated nanospheres†
Abstract
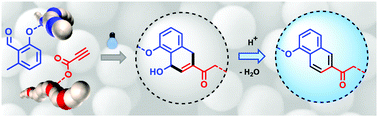
Herein, we introduce a fast, additive-free, ambient temperature photochemical approach – utilising the novel Diels–Alder cycloaddition of a photo-active ortho-methylbenzaldehyde (oMBA) with a terminal alkyne – for preparing functional acid-sensitive profluorescent nano-/microspheres in one step. Not previously reported, the possibility of applying such a reaction in the context of particle synthesis provides new possibilities for particle design, where multi-step reactivity can be gated into distinct steps. First, a photochemically-gated particle formation step yields a material possessing a reactive, spring-loaded intermediate at every cross-linking point. A second, on-demand step to initiate fluorescence generation subsequently imparts the properties of the chemical transformation to the material itself. The synthesised particles are narrow-disperse with an average diameter ranging from 170–380 nm.


 Please wait while we load your content...
Please wait while we load your content...