Controllable one-pot synthesis for scaffold diversity via visible-light photoredox-catalyzed Giese reaction and further transformation†
Abstract
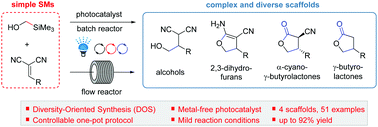
This study presents a controllable one-pot synthesis for constructing valuable scaffolds (alcohols, 2,3-dihydrofurans, α-cyano-γ-butyrolactones, and γ-butyrolactones) via a visible-light photoredox-catalyzed Giese reaction and further transformation. This one-pot reaction can selectively synthesize the desired scaffold in excellent yields with good functional group tolerance. To further highlight the broad applicability of this controllable one-pot reaction, we have established flow reaction conditions with short reaction times for the scale-up of each scaffold and demonstrated the further transformation of 2,3-dihydrofurans and α-cyano-γ-butyrolactones to achieve scaffold diversity for applications in drug discovery.


 Please wait while we load your content...
Please wait while we load your content...