Electrochemical detection of organophosphorus pesticides based on amino acids-conjugated P3TAA-modified electrodes
Abstract
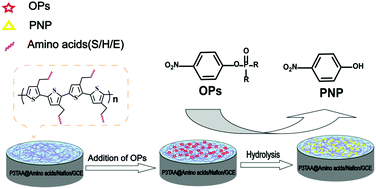
In this work, amino acids (AAs) including serine (S), histidine (H) and glutamic acid (E)-conjugated poly(3-thiophene acetate acid) (P3TAA) were synthesized to promote the catalytic hydrolysis and in situ electrochemical detection of organophosphorus pesticides (OPs). The hydrolysis of OPs followed the mechanism of proton transfer relay composed of AAs of S, H, E, called the “catalytic triad”, found in biomimetic hydrolases. P3TAA was used as a carrier to attach S, H, E, and these AA sites have the hydrolysis activity of Ops; the polymer P3TAA-AAs behaved like biomimetic enzymes. After the hydrolysis of OPs (e.g., methyl paraoxon, ethyl paraoxon and methyl parathion), p-nitrophenol (PNP) was generated, which can be detected electrochemically. Herein, an electrochemical method using P3TAA-conjugated S, H, E-modified electrodes for the determination of OPs was developed. OPs can be quantified by the electrochemical responses of PNP. This technique was selective toward OPs with the p-nitrophenol group. The detection limit of OPs (methyl paraoxon, methyl parathion and ethyl paraoxon) reached 0.5 μM. This detection technique was successfully applied to the detection of OPs in real samples with high detection accuracy.


 Please wait while we load your content...
Please wait while we load your content...