Facile catalyst-free synthesis, exchanging, and hydrolysis of an acetal motif for dynamic covalent networks†
Abstract
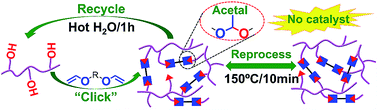
Dynamic covalent networks offer the favorable features of cross-linked polymers as well as the functions of reprocessing, recycling, self-healing, reshaping, and welding; however, it is a challenge to design readily monomer-recovery, highly malleable, catalyst-free, dynamic materials. Here we report the first design of acetal dynamic networks to address this challenge. Acetal dynamic networks were built via the catalyst-free “click” addition of polyol and a commercial divinyl ether without releasing small molecules. Small-molecule model compounds demonstrated thermally-induced acetal exchange without a catalyst. There are two mechanisms for the dynamic exchange of acetal: one is the metathesis of acetal, another is transacetalization. Acetal dynamic covalent networks exhibited excellent malleability and recyclability. They presented rapid stress relaxation at high temperatures. Hot press recovery can be achieved in 10 min at 150 °C. Meanwhile, the starting material was recovered with 92% recovery in 1 h under hot water treatment at 100 °C, and could be recross-linked with a commercial divinyl ether to obtain an acetal network. The acetal networks recovered by the two methods maintained the original structure and performance. An acetal dynamic linkage will open up a new way for the development of catalyst-free dynamic covalent networks and enrich acetal chemistry.


 Please wait while we load your content...
Please wait while we load your content...