Fine-tuning the coordination atoms of copper redox mediators: an effective strategy for boosting the photovoltage of dye-sensitized solar cells†
Abstract
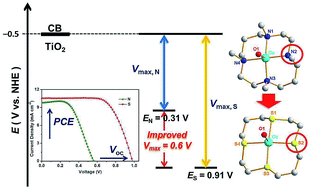
Natural systems have marvelously utilized copper complexes featuring sulfur-coordinating ligands, known as blue copper proteins, as efficient electron-transfer mediators in biological processes. Copper complexes with sulfur-coordinating ligands have been attempted as redox mediators in dye-sensitized solar cells (DSCs), the performance of which is not yet satisfactory and still remains less well explored. Herein, we report the application of new copper complexes bearing a tetradentate polythioether ligand, [(S4)Cu]2+/+ (12+/+, S4 = 1,4,8,11-tetrathiocyclotetradecane), as a redox mediator in DSCs in comparison with its N4-tetradentate counterpart [(N4)Cu]2+/+ (22+/+, N4 = 1,4,8,11-tetramethyl-1,4,8,11-tetraazacyclotetradecane). Impressively, the changes of coordination atoms from N to S positively shift the formal redox potential of the copper complexes by 600 mV, leading to a remarkably high photovoltage approaching 1.0 V. This is one of the highest photovoltage values reported thus far for DSCs based on copper redox mediators.


 Please wait while we load your content...
Please wait while we load your content...