Surface chemistry of tube-in-tube nanostructured cuprous sulfide@void@carbon in catalytical polysulfide conversion
Abstract
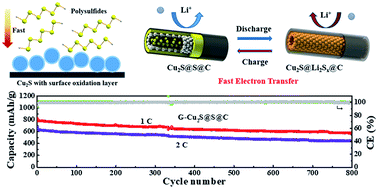
Polysulfide trapping to prevent the shuttle effect by a variety of polar materials has been intensively studied in Li–S batteries. However, the underlying chemical interaction and reaction kinetics remain poorly understood. Here, focusing on the fundamental understanding of the interfacial chemistry between sulfur cathode materials and lithium polysulfides, we designed and investigated a host material for sulfur, Cu2S@void@C, a tube-in-tube nanostructure with a polar Cu2S inner tube, conductive carbon outer tube and space in between. We found that the Cu2S nanotubes not only have strong adsorptivity to polysulfides through chemical interactions between the CuO surface layer and polysulfides, but also have a catalytic effect to lower the energy barrier of charge transfer at the polysulfides–electrolyte/electrode interface. The Cu2S nanotubes are able to enhance the reaction kinetics of polysulfide lithiation/delithiation and Li2S nucleation/oxidation. A binder free sulfur cathode (G–Cu2S@S@C) with an exquisite 3D conductive network was prepared by encapsulating sulfur into Cu2S@void@C and subsequently wrapping with graphene nanosheets. Remarkably, the G–Cu2S@S@C cathode with a 3.5 mg cm−2 sulfur loading amount shows a significantly prolonged cycling stability and enhanced rate performance.


 Please wait while we load your content...
Please wait while we load your content...