Polypyrrole-encapsulated amorphous Bi2S3 hollow sphere for long life sodium ion batteries and lithium–sulfur batteries†
Abstract
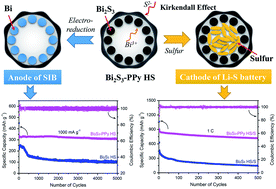
Sodium ion batteries (SIBs) and lithium–sulfur (Li–S) batteries are considered as the most promising next-generation energy storage devices to displace the widely used lithium ion batteries due to their inherent advantages. Here, polypyrrole-encapsulated amorphous Bi2S3 hollow spheres are prepared by the sulfuration of Bi–glycol spheres with a polypyrrole (PPy) coating. Benefiting from the unique hollow structure (Kirkendall effect) and the excellent conductivity and flexibility of the PPy coating, this material can be applied in both SIBs and Li–S batteries. For SIBs, the unconductive amorphous Bi2S3 is electrochemically reduced to conductive metallic Bi, leading to a Bi–PPy core–shell structure. The resulting Bi acts as the main active material while the PPy coating accommodates the volume variation of Bi during sodiation/desodiation. For Li–S batteries, the outer double layer structure effectively prevents the dissolution and “shuttle effect” of polysulfides by physically containing and chemically adsorbing polysulfides, and tolerates the volume expansion of sulfur. Meanwhile, the residual polyvinyl pyrrolidone in the preparation process is chemically bonded with sulfur and exhibits the electrochemical properties, and results in additional discharging/charging capacity. Thus, this multifunctional electrode shows excellent rate capability and cycling life in SIBs and Li–S batteries. This work provides new insight into the design of multifunctional electrodes.


 Please wait while we load your content...
Please wait while we load your content...