Highly effective fabrication of two dimensional metal oxides as high performance lithium storage anodes†
Abstract
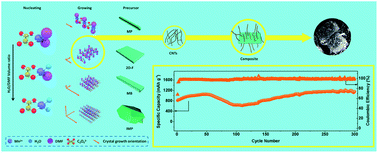
A facile strategy is proposed to control the morphology of manganese oxide (MnO), which is based on the ratio regulation of the solvents, N,N-dimethylformamide (DMF) and water, during nucleation and growth of the manganese oxalate precursor in solution. Various physical characterizations combined with theoretical calculations demonstrate that the crystal nucleation and growth of the precursor are highly dependent on the solvent composition, and thus the precursor as well as the resulting MnO can be controlled morphologically by regulating the ratio of water to DMF. Electrochemical measurements indicate that the sample with a two dimensional flake morphology exhibits excellent lithium storage performances in terms of specific capacity, cycling stability and rate capability, especially when carbon nanotubes (CNTs) are introduced during the precursor formation. The resulting composite of MnO and CNTs delivers a capacity of 1158 mA h g−1 after 300 cycles at 0.2 A g−1. Even at 2 A g−1, a remarkable capability of ∼630 mA h g−1 is achieved. When the composite is paired with a high-nickel cathode (NCA) in a full cell, an energy density of 426 W h kg−1 is harvested, indicative of their potential applications in next-generation lithium ion batteries.


 Please wait while we load your content...
Please wait while we load your content...