Supramolecular organization of membrane proteins with anisotropic hydrophobic thickness
Abstract
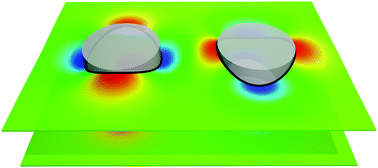
Experiments have revealed that membrane proteins often self-assemble into locally ordered clusters. Such membrane protein lattices can play key roles in the functional organization of cell membranes. Membrane protein organization can be driven, at least in part, by bilayer–mediated elastic interactions between membrane proteins. For membrane proteins with anisotropic hydrophobic thickness, bilayer–mediated protein interactions are inherently directional. Here we establish general relations between anisotropy in membrane protein hydrophobic thickness and supramolecular membrane protein organization. We show that protein symmetry is distinctively reflected in the energy landscape of bilayer–mediated protein interactions, favoring characteristic lattice architectures of membrane protein clusters. We find that, in the presence of thermal fluctuations, anisotropy in protein hydrophobic thickness can induce membrane proteins to form mesh-like structures dividing the membrane into compartments. Our results help to elucidate the physical principles and mechanisms underlying the functional organization of cell membranes.


 Please wait while we load your content...
Please wait while we load your content...