Enhanced low-temperature catalytic carbon monoxide methanation performance via vermiculite-derived silicon carbide-supported nickel nanoparticles
Abstract
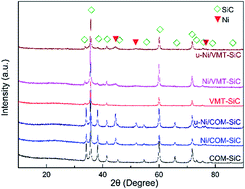
The methanation process is renowned worldwide and effectually employed for synthetic natural gas (SNG) production. It involves a highly exothermic reaction that can induce severe sintering of the catalyst and heavy carbon deposition, leading to catalyst deactivation. Thus, this process requires well-dispersed active metal catalyst components, improved thermal conductivity of the catalyst supports, and the lowest possible reaction temperature. Consequently, we successfully synthesized SiC ceramics with high specific surface areas and large pore volumes using vermiculite (VMT), and methanation catalysts comprising small Ni nanoparticles with mean diameter of 7.8 nm were produced on VMT-SiC supports by a conventional impregnation method. Contrastingly, Ni catalysts similarly prepared on commercially obtained SiC supports (Ni/VMT-SiC) demonstrated higher catalytic activity (CO conversion of 83.2% at 250 °C, pressure of 0.1 MPa, and gas hourly space velocity of 18 000 mL g−1 h−1) and stronger sintering resistance during long-term (50 h) low-temperature CO methanation experiments. Concisely, these improvements can be attributed to the superior thermal conductivity of SiC, high Ni dispersion and smaller Ni particle size, and strong metal–support interactions in the Ni/VMT-SiC catalysts. Moreover, we believe that this new insight will be worthy for future studies of CO methanation.


 Please wait while we load your content...
Please wait while we load your content...