Directed C(sp3)–H arylation of tryptophan: transformation of the directing group into an activated amide†
Abstract
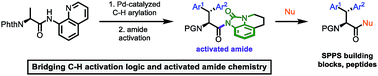
The 8-aminoquinoline (8AQ) directed C(sp3)–H functionalization was applied in the synthesis of β-arylated tryptophan derivatives. The laborious protecting group reorganization towards α-amino acids compatible for solid phase peptide synthesis (SPPS) was cut short by the transformation of the directing group into an activated amide, which was either used directly in peptide coupling or in the gram scale synthesis of storable Fmoc-protected amino acids for SPPS. In this work, directed C–H activation and nonplanar amide chemistry complement each other for the synthesis of hybrids between phenylalanine and tryptophan with restricted side chain mobility.


 Please wait while we load your content...
Please wait while we load your content...