Catalyst-controlled regiodivergent ring-opening C(sp3)–Si bond-forming reactions of 2-arylaziridines with silylborane enabled by synergistic palladium/copper dual catalysis†
Abstract
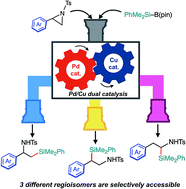
A catalyst-controlled regiodivergent and stereospecific ring-opening C(sp3)–Si cross-coupling of 2-arylaziridines with silylborane enabled by synergistic Pd/Cu dual catalysis has been developed. Just by selecting a suitable combination of catalysts, the regioselectivity of the coupling is completely switched to efficiently provide two regioisomers of β-silylamines (i.e., β-silyl-α-phenethylamines and β-silyl-β-phenethylamines) in good to high yields. Furthermore, a slight modification of the reaction conditions caused a drastic change in reaction pathways, leading to a tandem reaction to produce another regioisomer of silylamine (i.e., α-silyl-β-phenethylamines) in an efficient and selective manner.


 Please wait while we load your content...
Please wait while we load your content...