Palladium-catalyzed enantioselective alkenylation of alkenylbenzene derivatives†
Abstract
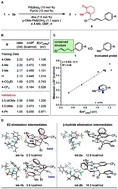
A regioselective and enantioselective palladium-catalyzed relay Heck alkenylation of alkenylbenzene derivatives to construct remote stereocenters is disclosed. Various β-substituted styrenes were readily obtained in moderate yields with good to excellent levels of enantioselectivity. This strategy provides rapid access to enantioenriched δ, ε, ζ, and η-alkenyl aryl compounds from simple starting materials. Mechanistic studies suggest that termination of the relay reaction is controlled by affinity of the arene for the Pd complex during migration.


 Please wait while we load your content...
Please wait while we load your content...