Substituent-controlled, mild oxidative fluorination of iodoarenes: synthesis and structural study of aryl I(iii)- and I(v)-fluorides†
Abstract
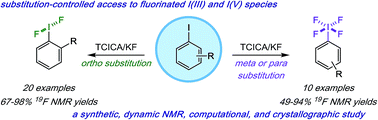
We report a mild approach to the synthesis of difluoro(aryl)-λ3-iodanes (aryl-IF2 compounds) and tetrafluoro(aryl)-λ5-iodanes (aryl-IF4 compounds) using trichloroisocyanuric acid (TCICA) and potassium fluoride (KF). Under these reaction conditions, selective access to either the I(III)- or I(V)-derivatives is predictable based solely on the substitution pattern of the iodoarene starting material. Moreover, the discovery of this TCICA/KF approach prompted detailed dynamic NMR, kinetic, computational, and crystallographic studies on the relationship between the IF2 group and the ortho-substituents on carefully designed probe molecules. It was during these experiments that the role of the ortho-substituent in inhibiting further oxidative fluorination of I(III)-compounds to I(V)-compounds during the reaction with TCICA and KF was revealed. Additionally, a notable exception to this empirical trend is discussed herein.


 Please wait while we load your content...
Please wait while we load your content...