Consecutive HDDA and TDDA reactions of silicon-tethered tetraynes for the synthesis of dibenzosilole-fused polycyclic compounds and their unique reactivity†
Abstract
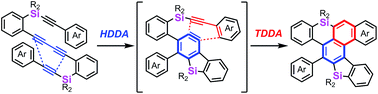
Silicon-tethered tetraynes possessing a 1,3-diyne moiety underwent consecutive hexadehydro- and tetradehydro-Diels–Alder reactions to give a series of fused polycyclic aromatic compounds containing a dibenzosilole skeleton. The benzene ring in the product acted as a 1,3-diene and reacted with the active alkyne as well as oxygen to provide [4 + 2] cycloadducts.


 Please wait while we load your content...
Please wait while we load your content...