Metal–ligand covalency enables room temperature molecular qubit candidates†
Abstract
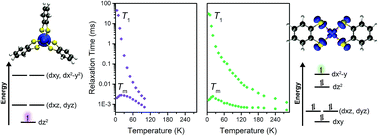
Harnessing synthetic chemistry to design electronic spin-based qubits, the smallest unit of a quantum information system, enables us to probe fundamental questions regarding spin relaxation dynamics. We sought to probe the influence of metal–ligand covalency on spin–lattice relaxation, which comprises the upper limit of coherence time. Specifically, we studied the impact of the first coordination sphere on spin–lattice relaxation through a series of four molecules featuring V–S, V–Se, Cu–S, and Cu–Se bonds, the Ph4P+ salts of the complexes [V(C6H4S2)3]2− (1), [Cu(C6H4S2)2]2− (2), [V(C6H4Se2)3]2− (3), and [Cu(C6H4Se2)2]2− (4). The combined results of pulse electron paramagnetic resonance spectroscopy and ac magnetic susceptibility studies demonstrate the influence of greater M–L covalency, and consequently spin-delocalization onto the ligand, on elongating spin–lattice relaxation times. Notably, we observe the longest spin–lattice relaxation times in 2, and spin echos that survive until room temperature in both copper complexes (2 and 4).


 Please wait while we load your content...
Please wait while we load your content...