Synthesis, cytotoxicity and anti-inflammatory activity of rhamnose-containing ursolic and betulinic acid saponins†
Abstract
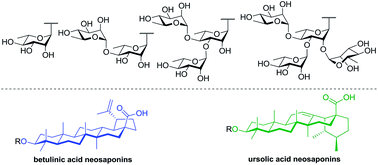
Betulinic acid and ursolic acid are ubiquitous, naturally-occurring triterpenoids exhibiting various pharmacological activities including cytotoxic and anti-inflammatory activities. However, these triterpenoids display unfavorable pharmacokinetic properties as well as low aqueous solubility. It has been shown that the presence of α-L-rhamnose moieties positively modulates the anticancer activity of secondary metabolites. Herein we report the synthesis and in vitro evaluation of cytotoxic and anti-inflammatory activities of a series of rhamnose-containing ursolic and betulinic acid saponins. Relying on Schmidt's normal and inverse procedures, monorhamnosides, (1→4)-linked dirhamnosides as well as branched trirhamnosides and tetrarhamnosides were synthesized in high yields with full control of stereoselectivity. A betulinic acid saponin bearing a 3-O-α-L-rhamnopyranosyl-(1→4)-α-L-rhamnopyranosyl residue was found to be a potent cytotoxic agent against human colorectal adenocarcinoma cells without damaging the healthy cells (selectivity ratio > 20) whereas rhamnose-containing ursolic acid saponins potently inhibited NO overproduction induced by LPS-stimulated macrophages. Our results reveal that rhamnose-containing ursolic and betulinic acid saponins represent promising therapeutic agents.


 Please wait while we load your content...
Please wait while we load your content...