Effect of the inserted active-site-covering lid loop on the catalytic activity of a mutant B. subtilis γ-glutamyltransferase (GGT)†
Abstract
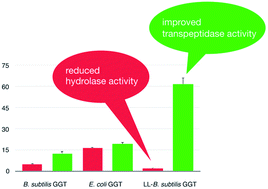
γ-Glutamylpeptides are compounds derived from the acylation of an amino acid or a short peptide by the γ-carboxyl carbon of the side chain of glutamic acid. Due to their altered chemico-physical and organoleptic properties, they may be interesting substitutes or precursors of parent compounds used in pharmaceutical, dietetic and cosmetic formulations. Some of them are naturally occurring flavor enhancers or are endowed with biological activities. Enzymatic approaches to the synthesis of γ-glutamyl derivatives based on the use of γ-glutamyltransferases (GGTs, EC 2.3.2.2) have been proposed, which should be able to alleviate the problems connected with the troublesome and low-yielding extraction from natural sources or the non-economical chemical synthesis, which requires protection/deprotection steps. With the aim of overcoming the current limitations in the use of GGTs as biocatalysts, a mutant GGT was investigated. The mutant GGT was obtained by inserting the active-site-covering lid loop of the E. coli GGT onto the structure of B. subtilis GGT. With respect to the wild-type enzyme, the mutant showed a more demanding substrate specificity and a low hydrolase activity. These results represent an attempt to correlate the structural features of a GGT to its different activities. However, the ability of the mutant enzyme to catalyze the subsequent addition of several γ-glutamyl units, inherited by the parent B. subtilis GGT, still represents a limitation to its full application as a biocatalyst for preparative purposes.


 Please wait while we load your content...
Please wait while we load your content...