Gas-phase UV absorption spectra and OH-oxidation kinetics of 1H-1,2,3-triazole and pyrazole†
Abstract
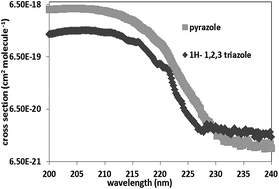
In this work, we report the gas phase UV absorption spectra and the kinetics of the OH-oxidation of 1H-1,2,3-triazole and pyrazole. UV spectra were determined between 200 and 250 nm, at 350 ± 2 K and at pressures between 0.09 and 0.3 Torr. The reported maximal UV absorption cross sections are (cm2 per molecule): σ206 nm, 1H–1H-1,2,3-triazole = 2.04 × 10−18 and σ203 nm, pyrazole = 5.44 × 10−18. The very low absorption capacity of these compounds beyond 240 nm indicates that their atmospheric photodissociation is negligible. The OH-oxidation of these species was performed in an atmospheric simulation chamber coupled to an FTIR spectrometer and to a GC/MS over the temperature range 298–357 K and at atmospheric pressure. Experiments were conducted in relative mode using benzaldehyde, trans-2-hexenal and heptane as references. The obtained rate constants at 298 K were (×10−11 cm3 per molecule per s): k(OH + 1H-1,2,3-triazole) = 2.16 ± 0.41; k(OH + pyrazole) = 2.94 ± 0.42. These results were compared to those available in the literature and discussed in terms of structure-reactivity and temperature dependency. Their tropospheric lifetimes with respect to reaction with OH radicals were then estimated.


 Please wait while we load your content...
Please wait while we load your content...