Synthesis and biological activities of petrosiols B and D†
Abstract
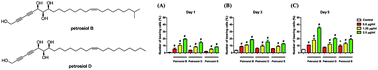
A divergent total synthesis of natural diacetylenic tetraols, petrosiol B and petrosiol D, was accomplished by taking advantage of a carbohydrate chiral template. In particular, petrosiol B, which is the first total synthesis so far, was achieved in 13 linear steps with a 10% overall yield applying Ohira–Bestmann homologation, NaH-mediated dehydrobromination, and Cu(I)-catalyzed Cadiot–Chodkiewicz coupling as the key reaction steps. The synthetic petrosiols B and D were subjected to the study on differentiation activities toward neuronal progenitor PC12 cells. Our results suggested that both petrosiol B and petrosiol D could induce the differentiation of neuronal progenitor PC12 cells via the enhancement of Nrf2 activity. By comparing petrosiols B, D and their natural homologue E, petrosiol B displayed the most intensive cell differentiation activity and the highest Nrf2 activity enhancement as well.


 Please wait while we load your content...
Please wait while we load your content...