Synthesis, characterization and chemical degradation of poly(ester-triazole)s derived from d-galactose†
Abstract
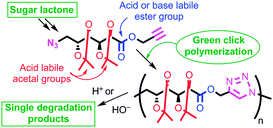
α-Azide-ω-alkynyl ester monomers were designed and synthesized in order to obtain hydrolytically degradable polymers. The monomers were prepared from D-galactose, as a renewable resource. Environmentally benign azido–alkyne cycloaddition polymerizations were conducted to afford poly(ester-triazole)s, with complete atom economy. Although polymer formation prevailed under optimized polymerization conditions, variable proportions of cyclic oligomer byproducts were detected. The Cu-catalyzed click polymerization led regioselectively to 1,4-disubstituted triazole linkages, while the thermal, metal-free polymerization produced a random distribution of 1,4- and 1,5-disubstituted triazoles in the polymer backbone. The poly(ester-triazole)s exhibited high molecular weights (Mw in the range 35–85 kDa). They were soluble in organic solvents but highly insoluble in water, thus removal of the Cu(I) catalyst was simplified. The polymers were stable up to 300 °C, and had Tg values in the range 90–100 °C. The materials were hydrolysed under either basic or strong acid conditions, and the degradation products have been characterized.


 Please wait while we load your content...
Please wait while we load your content...