Mechanisms of isomerization and oxidation in heated trilinolein by DFT method†
Abstract
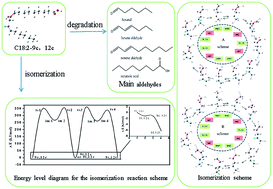
In order to investigate the molecular mechanisms of the heat-induced cis/trans isomerization and oxidative cleavage of trilinolein, a highly purified sample was heated at a range of temperatures (120, 140, 160, 180, 200, 220 °C) for 5 h. The reaction process of cis/trans isomerization of C18:2 was studied by a combination of the gas chromatogram (GC) method with density functional theory (DFT). When trilinolein was heated to 180 °C, a small amount of trans/trans C18:2 was obtained (0.074 mg g−1). As the temperature increased to 220 °C, the amount of trans C18:2 reached 0.198 mg g−1. This study shows that C18:2-9c12t and C18:2-9t12c were the main trans fatty acids in heated trilinolein. The molecular mechanisms of isomerization and oxidative cleavage were verified by Gaussian 09 W software. All the geometry was optimized using DFT at the B3LYP/6-31*G level. The energy difference between cis and trans linoleic acid was equal to 6.2 kJ mol−1. Therefore, vegetable oil with a higher linoleic acid content should be maintained at 140 °C or less to avoid the formation of trans linoleic acid.


 Please wait while we load your content...
Please wait while we load your content...