Continuous flow synthesis of diaryl ketones by coupling of aryl Grignard reagents with acyl chlorides under mild conditions in the ecofriendly solvent 2-methyltetrahydrofuran†
Abstract
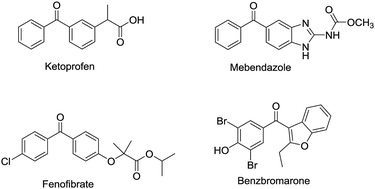
An efficient continuous flow sequential synthesis of diaryl ketones was achieved by coupling of aryl Grignard reagents with acyl chlorides in the bio-derived “green” solvent 2-methyltetrahydrofuran (2-MeTHF) under mild reaction conditions (ambient temperature, 1 hour), allowing a safe and on-demand generation of 2-(3-benzoylphenyl)propionitrile with a productivity of 3.16 g hour−1.


 Please wait while we load your content...
Please wait while we load your content...