Ring-opening C(sp3)–C coupling of cyclobutanone oxime esters for the preparation of cyanoalkyl containing heterocycles enabled by photocatalysis†
Abstract
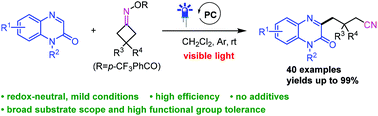
Here we report a ring-opening C(sp3)–C coupling of cyclobutanone oxime esters for the preparation of cyanoalkyl containing heterocycles under visible-light or sunlight irradiation. The reaction protocol is operationally simple and proceeds at room temperature without extra additives, allowing access to a variety of functionalized alkylnitriles in moderate to excellent yields. This transformation shows wide functional group compatibility and can be easily scaled up. A mechanism study discloses that the cyanoalkyl radical is involved in the present reaction.


 Please wait while we load your content...
Please wait while we load your content...