Visible-light-induced oxidative ring expansion of indoles with amidines†
Abstract
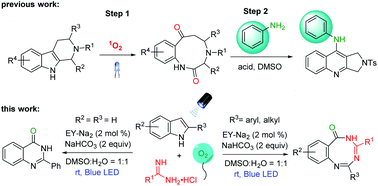
An efficient and mild visible-light-enabled reaction involving the oxidative ring expansion of indoles with amidines in the aqueous phase at room temperature is developed. Benzo[1,3,5]triazocin-6(5H)-ones and quinazolinones were facilely obtained as the terminal products by using 2-substituted indoles and substituent-free indoles as substrates, respectively. This protocol features mild conditions, excellent functional group tolerance, and moderate to good yields.


 Please wait while we load your content...
Please wait while we load your content...