Computational and experimental studies on Cu/Au-catalyzed stereoselective synthesis of 1,3-disubstituted allenes†
Abstract
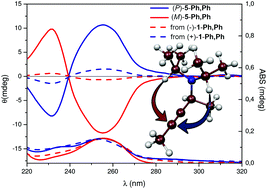
Cu- and Au-mediated formation of allenes from terminal alkynes and aldehydes via propargylamine intermediates is hampered by reversibility in the propargylamine formation. The use of a stable Au(I) catalyst in the reaction using a chiral propargylamine provided clues to disentangle the mechanism of the whole process that would have been otherwise hidden. Additionally, the process was observed to be stereoselective when an enantiomerically pure chiral propargylamine was used as starting substrate providing the corresponding 1,3-disubstituted allenes with high enantiomeric ratio.


 Please wait while we load your content...
Please wait while we load your content...