The effects of the heteroatom and position on excited-state intramolecular proton transfer of new hydroxyphenyl benzoxazole derivatives: a time-dependent density functional theory study
Abstract
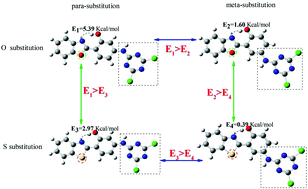
The effects of the heteroatom and position on excited-state intramolecular proton transfer (ESIPT) of 2-[4′-(N-4,6-dichloro-1,3,5-triazi-n-2-yl)2′hydroxyphenyl]benzoxazole (4THBO) have been investigated via time-dependent density functional theory studies. The heteroatoms refer to O and S atoms, and the position effect refers to the N-4,6-trichloro-1,3,5-triazin-2-yl (TCT) substituents in the para and meta positions. The configuration of the four compounds (4THBO, 4THBT, 5THBO and 5THBT) was optimized and the bond lengths, bond angles and infrared spectra of the atoms participating in the proton transfer in the S0 and S1 states were studied. The occurrence of ultrafast ESIPT in the four compounds was demonstrated. Moreover, the potential energy curves of the S0 and S1 states were constructed, and the effects of the heteroatom substitution and substituent position changes on the ESIPT mechanism of the four 4THBO derivatives were analyzed. The results show that the ESIPT barrier of the S atom substitution in the excited state is lower than that of the O atom-substituted molecule, and the energy barrier of the substituent (TCT) in the meta-position is significantly smaller than that in the para-position. These results indicate that the substitution of the S heteroatom promotes the ESIPT of the 4THBO compound and that the substituent (TCT) in the para position is more prone to proton transfer than that in the meta position. Our work could provide a theoretical basis for further experiments.


 Please wait while we load your content...
Please wait while we load your content...