Organocatalytic asymmetric synthesis of highly functionalized spiro-thiazolone–cyclopropane-oxindoles bearing two vicinal spiro quaternary centers†
Abstract
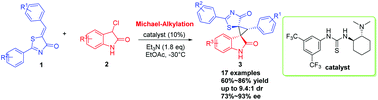
An effective organocatalytic asymmetric Michael/alkylation cascade reaction of 5-alkenyl thiazolones with 3-chloroxindoles catalyzed by Takemoto's bifunctional aminothiourea catalyst was developed. A variety of highly functionalized spiro-thiazolone–cyclopropane-oxindoles with three contiguous stereocenters, including two vicinal quaternary centers, were obtained in good yields (60%–86%) with moderate to good diastereoselectivities (up to 9.4 : 1 dr) and good enantioselectivities (up to 93% ee).


 Please wait while we load your content...
Please wait while we load your content...