Catalytic enantioselective cross-dehydrogenative coupling of 3,6-dihydro-2H-pyrans with aldehydes†
Abstract
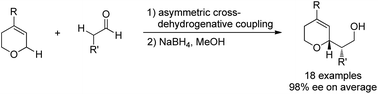
The existing catalytic asymmetric synthesis of enantiopure α-substituted 3,6-dihydro-2H-pyrans and tetrahydropyrans predominantly relies on the oxygen heterocycle construction strategy involving enantioselective cyclization of pre-functionalized olefin substrates. Given the easy availability of the cyclic ether skeleton, we disclosed the first catalytic asymmetric cross-dehydrogenative coupling of 3,6-dihydro-2H-pyrans and aldehydes. The reaction exhibits excellent enantioselectivity, good functional group tolerance, and wide compatibility of diverse 3,6-dihydro-2H-pyran and aldehyde components, thus providing a practical and economical method for enantiopure α-substituted 3,6-dihydro-2H-pyran synthesis.


 Please wait while we load your content...
Please wait while we load your content...