A mechanistic investigation into N-heterocyclic carbene (NHC) catalyzed umpolung of ketones and benzonitriles: is the cyano group better than the classical carbonyl group for the addition of NHC?†
Abstract
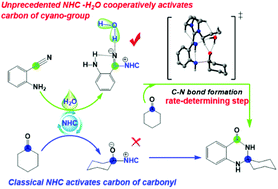
Density functional theory (DFT) computations have been utilized to investigate the N-heterocyclic carbene (NHC) catalytic reaction mechanism when carbonyl and cyano compounds co-exist. The results suggested that both the carbonyl and cyano compounds can be activated by NHC, while the order of activation by the NHC depends on the substituents of the two compounds. In the model reaction of NHC catalyzed cyclocondensation of cyclohexanone with 2-aminobenzonitrile, two key activation modes were explored, the new NHC activation carbon of the cyano-group (Mode II) is found to be perferred over the activation of carbonyl carbon (Mode I); this is remarkably at variance from the previous reports. More importantly, water molecules can improve the catalytic activity of NHC to some extent, especially in the Dimroth rearrangement process. The cooperative participation of the NHC and H2O catalyst is identified. Moreover, the strong quadruple hydrogen bonding interactions between water/NHC and substrates are proposed to be the origin of the high activity of NHC–H2O co-catalyzed Mode II. Additionally, these interactions stabilize the generated negative charge on carbonyl oxygen and further strengthen the nucleophilicity of the –NH group in the NHC–H2O co-catalyzed key transition state structure, which is further confirmed by AIM and NCI analyses. This novel preferred umpolung of a cyano-group via NHC–H2O catalysis was proposed to be responsible for the experimentally observed high product yield.


 Please wait while we load your content...
Please wait while we load your content...