A computational study of cobalt-catalyzed C–H iodination reactions using a bidentate directing group with molecular iodine†
Abstract
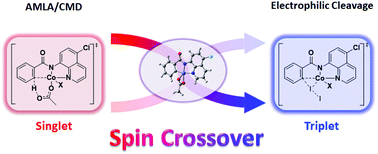
A computational methodology was used to collect detailed mechanistic information on the cobalt-catalyzed C–H iodination of aromatic amides with molecular iodine using an N,N′-bidentate directing group. Based on the calculations, the C–H activation step proceeds through a singlet state concerted metalation–deprotonation (CMD) pathway through an agostic intermediate, and a single electron transfer (SET) pathway was found to be theoretically impossible. A strong correlation between singlet–triplet energy gaps and the spin densities of the cobalt atom is observed. We also studied the impact of ligands on the CMD pathway. The iodination step proceeds via a triplet state redox-neutral electrophilic cleavage (EC) pathway. The cobalt catalyst in C–H activation reactions exists in complex electronic states, and experimental mechanistic studies such as low kinetic isotope effects and radical scavenger inhibition results may be misleading with respect to the actual mechanism for such reactions.


 Please wait while we load your content...
Please wait while we load your content...