N,N′-Substituted acryloamidines – novel comonomers for melt-processible poly(acrylonitrile)-based carbon fiber precursors†
Abstract
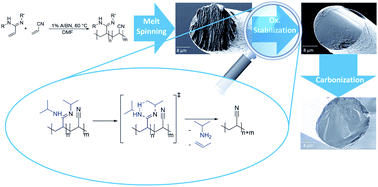
To date, carbon fibers (CFs) are mostly derived from poly(acrylonitrile) (PAN) precursor fibers, which are processed via solution spinning. In this study, a novel, melt processible class of PAN-based CF precursors is presented. The polymeric precursors are synthesized via copolymerization of acrylonitrile with N,N′-substituted acryloamidines. Analysis of the volatile compounds formed during cyclization strongly suggests the reformation of the nitrile group via concomitant formation of primary amines. In air, the copolymers inherit a very low onset temperature of cyclization, Tonset-c, of 138 °C in case 20 mol% of N,N′-di(2-propyl)acryloamidine are incorporated. Copolymerization parameters were determined and suggest an almost statistical incorporation of both comonomers. Both the thermal and rheological properties of the copolymers have been studied and allow for melt spinning. Melt spun fibers were transformed into CFs via stabilization in air followed by carbonization under nitrogen. CF formation was followed up to 1800 °C by wide-angle X-ray scattering and Raman analysis.


 Please wait while we load your content...
Please wait while we load your content...