Effects of incorporated pyrazine on the interchain packing and photovoltaic properties of wide-bandgap D–A polymers for non-fullerene polymer solar cells†
Abstract
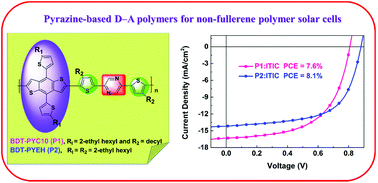
Great advancements in the photovoltaic performance of polymer solar cells (PSCs) have been achieved through the synergistic effect of aromatic and heteroaromatic units incorporated into the backbone of a conjugated polymer. However, efficient donor polymers with electron-deficient nitrogen heterocycles (N-heterocycles) for PSCs have been relatively less explored in terms of number and diversity relative to thienoacene counterparts. In this report, we investigated, for the first time, pyrazine as an acceptor core for designing wide-bandgap (WBG) polymer donors poly-{2-(5-(4,8-bis(5-(2-ethylhexyl)thiophen-2-yl)-6-methylbenzo[1,2-b:4,5-b′]dithiophen-2-yl)-4-decylthiophen-2-yl)-5-(4-decyl-5-methylthiophen-2-yl)pyrazine} (P1) and poly-{2-(5-(4,8-bis(5-(2-ethylhexyl)thiophen-2-yl)-6-methylbenzo[1,2-b:4,5-b′]dithiophen-2-yl)-4-(2-ethylhexyl)thiophen-2-yl)-5-(4-(2-ethylhexyl)-5-methylthiophen-2-yl)pyrazine} (P2) having different alkyl side chains for use in non-fullerene (NF) PSCs. These polymers demonstrated deep frontier energy levels and wide bandgaps (∼2.05 eV) because of the electron-withdrawing ability of the nitrogen atoms in their pyrazine core. Additionally, they also demonstrated stronger aggregation and enhanced coplanarity because of the noncovalent interaction between the nitrogen atom of the pyrazine and adjacent thiophene units. The optimized PSCs obtained by blending P1 and P2 with an NF acceptor, 3,9-bis(2-methylene-(3-(1,1-dicyanomethylene)-indanone))-5,5,11,11-tetrakis(4-hexylphenyl)-dithieno[2,3-d:2′,3′-d′]-s-indaceno[1,2-b:5,6-b′]dithiophene (ITIC) exhibited impressive efficiencies of 7.6 and 8.1%, respectively. The observed variation in the performance was studied by various characterization methods. The superior photovoltaic performance of the P2 : ITIC blend originated from its distinctly optimized polymer design via regulation of its alkyl side chains, which led to downshifted frontier energy levels, a face-on orientation, and an optimum morphology compared with those of the P1 : ITIC blend. Our findings advance the understanding of the influence of incorporated pyrazine on the optoelectronic properties of polymer donors, and this structure–relationship study provides valuable insights for designing highly efficient copolymers with N-heterocycles.


 Please wait while we load your content...
Please wait while we load your content...