Catalytic enantioselective Michael addition of 2-substituted benzofuran-3-ones to 2-enoyl pyridines†
Abstract
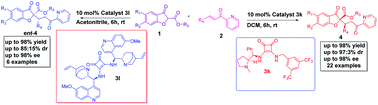
An organocatalytic diastereo- and enantioselective synthesis of 2,2′-disubstituted benzofuran-3-ones bearing adjacent quaternary and tertiary stereocenters has been achieved through Michael addition of 2-substituted benzofuran-3-ones to 2-enoyl pyridines. Both the enantiomeric forms of the major diastereomer were obtained using L-proline derived squaramide and quinine derived bis squaramide with excellent yield (up to 98%) and stereoselectivities (up to 97 : 3 dr and 98% ee). The control experiment revealed that the presence and position of nitrogen atoms in the 2-enoylpyridine have played a crucial role in the stereochemical outcome of the product.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...