Catalytic direct amidations in tert-butyl acetate using B(OCH2CF3)3†
Abstract
Catalytic direct amidation reactions have been the focus of considerable recent research effort, due to the widespread use of amide formation processes in pharmaceutical synthesis. However, the vast majority of catalytic amidations are performed in non-polar solvents (aromatic hydrocarbons, ethers) which are typically undesirable from a sustainability perspective, and are often poor at solubilising polar carboxylic acid and amine substrates. As a consequence, most catalytic amidation protocols are unsuccessful when applied to polar and/or functionalised substrates of the kind commonly used in medicinal chemistry. In this paper we report a practical and useful catalytic direct amidation reaction using tert-butyl acetate as the reaction solvent. The use of an ester solvent offers improvements in terms of safety and sustainability, but also leads to an improved reaction scope with regard to polar substrates and less nucleophilic anilines, both of which are important components of amides used in medicinal chemistry. An amidation reaction was scaled up to 100 mmol and proceeded with excellent yield and efficiency, with a measured process mass intensity of 8.
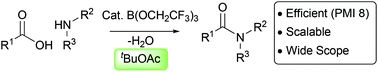
- This article is part of the themed collections: Catalysis & biocatalysis in OBC and Trends in Organoboron Chemistry


 Please wait while we load your content...
Please wait while we load your content...