Metal-free oxysulfonylation and aminosulfonylation of alkenyl oximes: synthesis of sulfonylated isoxazolines and cyclic nitrones†
Abstract
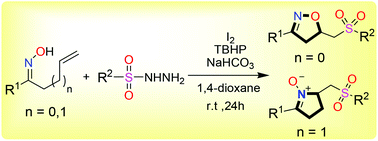
Intramolecular oxysulfonylation of alkenyl oximes was reported. Using iodine as the catalyst, TBHP as the oxidant, and sulfonyl hydrazides as the sulfonyl radical source, a variety of sulfonylated isoxazolines were obtained in moderate to excellent yields. Cyclic nitrones could also be readily obtained under the same conditions.


 Please wait while we load your content...
Please wait while we load your content...