Facile synthesis of PdFe alloy tetrahedrons for boosting electrocatalytic properties towards formic acid oxidation†
Abstract
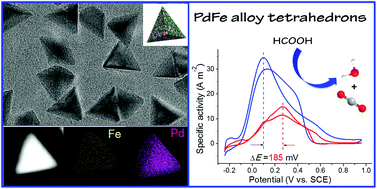
The controllable synthesis of multi-metal nanocrystals with a tetrahedral shape is significant for constructing high-efficiency electrocatalysts. However, due to the great distinction among the thermodynamic reduction potentials of different metal precursors, it is difficult to achieve tetrahedron-shaped alloy nanocrystals with a uniform {111} crystal surface and low surface energy. Herein, we reported a one-pot hydrothermal synthetic strategy to achieve high-yield PdFe alloy tetrahedrons. The unique structure endowed an impressive surface area-to-volume ratio, well distribution of Pd and Fe sites, and essential electronic effects, due to which they could be employed as formic acid oxidation reaction (FAOR) catalysts. As expected, the PdFe alloy tetrahedrons exhibited 4.8 and 2.4 times higher mass activity (595.8 A g−1) and specific activity (33.4 A m−2) compared to commercial Pd black, respectively; they also showed enhanced electrocatalytic stability and good resistance to CO poisoning. This work demonstrates the potential applications of bimetal Pd-based tetrahedrons as promising anode catalysts in a direct formic acid fuel cell.


 Please wait while we load your content...
Please wait while we load your content...