Low-temperature pseudomorphic transformation of polyhedral MIL-88A to lithium ferrite (LiFe3O5) in aqueous LiOH medium toward high Li storage†
Abstract
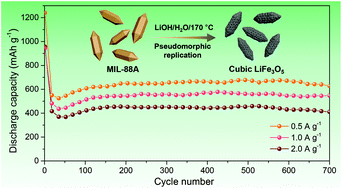
The ability to develop novel nanomaterials, and to precisely manufacture their functional structures at the nano- and microscales would benefit many emerging device applications. Herein, as a first example, we describe the exploration of feasibility for the morphological replacement of an iron-based MOF bearing trimeric FeIII–O clusters, MIL-88A preform, with a polyhedral architecture of around 0.4 × 1.2 μm by a lithium ferrite (LiFe3O5) phase via solid–liquid pseudomorphic transformation reactions in biologically and environmentally favourable aqueous lithium hydroxide (LiOH). The reaction proceeds at 170 °C, and the overall reaction can be described as Fe3O(H2O)2(FMA)3(OH)·nH2O (MIL-88A) + 7OH− + Li+ → LiFe3O5 + 3FMA2− + (n + 6) H2O (FMA = fumarate). It was proposed that through the coordination substitution of a MOF ligand by OH−, follow-up dehydration and dehydroxylation, and final H+/Li+ ionic exchange, the monolithiated iron oxides formed thermodynamically at comparatively low temperatures, which transcribe the global nanostructure morphologies of the polyhedral MOF preforms with the hexagonal symmetry, but were composed of interconnected LiFe3O5 particles (about 16 nm) that crystallize in a typical magnetite-type cubic (Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m) structure. Given the characteristic texture and structure of the Li–Fe oxide replica, cubic LiFe3O5 was preferentially employed as a new type of electrode material in rechargeable lithium cells. Notably, from the electrochemical evaluation, this metal oxide system exhibits decent anodic performances by undergoing a nine-electron conversion reaction, showing a substantially high specific capacity with an average potential of 0.8 V versus lithium metal, a long service life (700 cycles), and exceptional high-rate capability (up to 2.0 A g−1). The synthetic paradigms demonstrated that the MIL-88A to LiFe3O5 conversion may be transferable to other advanced inorganic-based electrodes from the parent metal compound such as LiFeO2, LiMn2O4 or LiCoO2 toward sustainable energy fields.
m) structure. Given the characteristic texture and structure of the Li–Fe oxide replica, cubic LiFe3O5 was preferentially employed as a new type of electrode material in rechargeable lithium cells. Notably, from the electrochemical evaluation, this metal oxide system exhibits decent anodic performances by undergoing a nine-electron conversion reaction, showing a substantially high specific capacity with an average potential of 0.8 V versus lithium metal, a long service life (700 cycles), and exceptional high-rate capability (up to 2.0 A g−1). The synthetic paradigms demonstrated that the MIL-88A to LiFe3O5 conversion may be transferable to other advanced inorganic-based electrodes from the parent metal compound such as LiFeO2, LiMn2O4 or LiCoO2 toward sustainable energy fields.


 Please wait while we load your content...
Please wait while we load your content...