A photoacoustic shockwave triggers the size shrinkage of nanoparticles to obviously improve tumor penetration and therapeutic efficacy†
Abstract
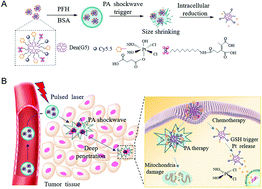
Drug delivery to a tumor site with an insufficient microvascular network remains a challenge due to the size preference for transport in terms of circulation and distribution. In this work, an integrated nano-therapeutic parcel disintegrable by a photoacoustic shockwave was developed. Nano-therapeutic particles with red absorbance are packaged into a larger parcel to generate a longer circulation half-life and improved accumulation in tumor tissue. Pulse-laser irradiation is absorbed by the nanoparticles and it generates a photoacoustic shockwave. This triggers a liquid–gas phase transition of the nano-parcel, leading to the high-efficiency release of smaller nanoparticles, thus achieving excellent therapeutic diffusion with improved uniformity. This results in a highly effective therapeutic effect, as demonstrated with both in vitro and in vivo tumor models. Compared to previously reported work, this approach has the distinctive advantage of precisely controllable therapeutic release that is independent of the physiological environment in the tumor and it is less limited than a UV-based release mechanism. In addition, the concept of photoacoustic shockwave-based nanoparticle release can be extended over a wide wavelength range, including microwaves, to match specific needs and achieve optimal therapeutic depth. The results demonstrate that the proposed strategy holds great potential for improved tumor therapy efficacy.


 Please wait while we load your content...
Please wait while we load your content...